How Many Outer Ring Electrons Does Chlorine Have
In a picture the valence electrons are the ones in the outermost shell. I know that if you want to figure out how many electrons an atom has on its outer ring valence its 1 for group 1 eg Na 2 for group 2 eg Mg all the way to 7 for halogens and 8 for noble gases.

See The Electron Configuration Diagrams For Atoms Of The Elements Atom Diagram Electron Configuration Atom Project
What are the symptoms of RAO.

. What forms are three-centre four-electron bonds one pair of electrons from the chlorine two single electrons from two fluorine atoms. The chlorine atom has a total of 17 electrons so we have to put 17 electrons in orbitals. That means there are 17 electrons in a chlorine atom.
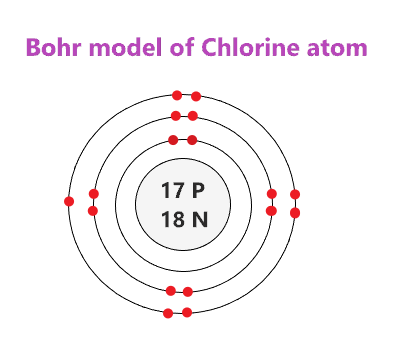
What does chlorine look like. It is now referred to as a. Chlorine has 17 electrons.
Electrons equal to protons are located in a circular shell outside the nucleus. Metals will ionically bond with chlorine and yield an electron to halogens forming a stable octet. 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f.
How many electrons does xenon have How many valence electrons does sodium have. We can see the outer most shell of chlorine has 7 electrons so have to subtract it from 8. Thats why valency of chlorine is 1.
However how do you figure it. Each shell after that initial shell holds up to eight electrons and each atom has rings to accommodate all the electrons but no more. That is a chlorine atom has a total of seventeen electrons.
Looking at the picture you can see there are two electrons in shell one eight in shell two and seven in shell three. It has 1 on the first ring and then it has 2 on the second ring and lithium has 2 rins for the atom How many electrons are in the third ring of a. While the transition elements in the sub shell n-1 d.
Hydrogen doesnt have enough shells to require 8 so its happy with just 2. Chlorine is in column 7 immediately before the noble gases. Chlorine is in column 7 immediately before the noble gases.
How many outer ring electrons does chlorine have. Therefore it tends to gain an electron to create an ion with 17 protons 17 neutrons and 18 electrons giving it a net negative 1 charge. Cl has 7 outer electrons and a full shell would correspond to having 8 outer electrons.
Now Trayvon Answeregy Expert How many valence electrons does a chloride ion Cl - have. Valence electrons are electrons used in forming bonds and are in the outer shell of an element. It is a pale green gas.
8 7 1. As for the symbol remember that an atom wants 8 electrons in its outer ring if possible. To do this it will more likely gain 1 than lose 7 so it will be a Chlorine ion with an extra electron.
Therefore the first ring is filled with two electrons the second ring is filled with eight electrons but there are only seven electrons for the third ring. The 3s23p5 electrons are the outermost electrons so chlorine has seven valence electrons. The electrons will be placed in different orbitals according to the energy level.
In general chlorine has a stable oxidation state of -1 most of the time but it can differ in some compounds with values 0 Cl2 1 NaClO 3 NaClO2 4 ClO3 5 NaClO3 7 NaClO4. Chlorine Cl in its lowest energy state called the ground state has seven electrons in its outer shell. This is because halogens have seven outer ring electrons valence electrons but need eight to form a stable configuration.
The number of neutrons in an element is obtained from the difference between the number of atomic masses and the number of atoms. The number of waves that pass a given point in one second. There are 2 options Lose 7 electrons - which does NOT happen because of the VAST amount of energy this would need or Gain one electron which is relatively easy and would help out another metal atom that wants to lose an electron win-win situation.
It is eager to give up this electron in order to have a full outer energy level because this will give it the most stable arrangement of electrons. Sodium chlorine and oxygen atoms all want outer shells to have 8 electrons. The main group elements usually use electrons in the subshell ns and np.
As a member of the halogen family on the Periodic Table chlorine is very reactive with metals and forms salts. 52 7. Theyre most stable and thus most happy with a full outer shell.
This is the halogen group and all of them have 7 valence electrons hence the column. The electron configuration of chlorine is 1s22s22p63s23p5 or Ne3s23p5. Howmany electrons fill the outer shell of carbon.
Sets found in the same folder. How many outer ring electrons does chlorine have. Carbon Cstart message C end message en masse 14 component has 4 electrons in its outershell Carbon generally shares electrons to attain a full valence shell generating bonds with many various various other atoms.
An atom of a group 1 element such as sodium has just one valence electron. You could also do this just by looking at the periodic table. Video Answer Ring and Forks Calculator Ring and Forks Calculator.
Chlorine atoms have two electrons in the inner ring then eight electrons in the second ring then seven electrons in their outer ring. On earth chlorine forms molecules of two chlorine atoms. How many rings does chlorine have.
Chlorine has 17 electrons. You can see in the diagram below that there are seven electrons in the outermost circle. 188166242150 We have shown the Valence Electrons of the elements for which reliable data is available.
What is Susans maximum affordable payment. New questions in Physics. She has located a lender that will qualify her using a 28 ratio.
Does a good insulator prevent heat from getting through it or does it simply delay its passage. This is the halogen group and all of them have 7 valence electrons hence the column. As for the symbol remember that an atom wants 8 electrons in its outer ring if possible.
For the element of CHLORINE you already know that the atomic number tells you the number of electrons. Susan earns 78000 per year. Chlorine has 10 core electrons.
Thats why interhalogens always have an uneven number of fluorine atoms except for the dimer C l X 2 F X 6. Therefore the first ring is filled with two electrons the second ring is filled with eight electrons but there are only seven electrons for the third ring. What are the 7 miracles in John.

Chlorine Science Model Atom Model Science Models Atom Model Project

Potassium Atom Showing Electrons In Their Shells Gcse Chemistry Electrons Chemistry
Chlorine Bohr Model How To Draw Bohr Diagram For Chlorine Cl

Comments
Post a Comment